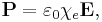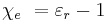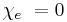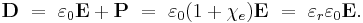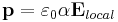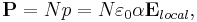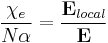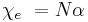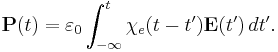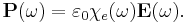Electric susceptibility
In electromagnetism, the electric susceptibility 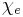 (latin: susceptibilis “receptiveness”) is a dimensionless proportionality constant that indicates the degree of polarization of a dielectric material in response to an applied electric field. The greater the electric susceptibility, the greater the ability of a material to polarize in response to the field, and thereby reduce the total electric field inside the material (and store energy). It is in this way that the electric susceptibility influences the electric permittivity of the material and thus influences many other phenomena in that medium, from the capacitance of capacitors to the speed of light.[1][2]
(latin: susceptibilis “receptiveness”) is a dimensionless proportionality constant that indicates the degree of polarization of a dielectric material in response to an applied electric field. The greater the electric susceptibility, the greater the ability of a material to polarize in response to the field, and thereby reduce the total electric field inside the material (and store energy). It is in this way that the electric susceptibility influences the electric permittivity of the material and thus influences many other phenomena in that medium, from the capacitance of capacitors to the speed of light.[1][2]
Contents |
Definition of Volume Susceptibility
Electric susceptibility is defined as the constant of proportionality (which may be a tensor) relating an electric field E to the induced dielectric polarization density P such that:
Where:
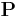 is the Polarization Density
is the Polarization Density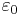 is the Electric Permittivity of Free Space
is the Electric Permittivity of Free Space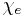 is the Electric Susceptibility
is the Electric Susceptibility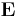 is the Electric Field
is the Electric Field
The susceptibility is also related to the polarizability of individual particles in the medium by the Clausius-Mossotti relation. The susceptibility is related to its relative permittivity 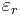 by:
by:
So in the case of a vacuum:
At the same time, the electric displacement D is related to the polarization density P by:
Molecular Polarizability
A similar parameter exists to relate the magnitude of the induced dipole moment p of an individual molecule to the local electric field E that induced the dipole. This parameter is the molecular polarizability and the dipole moment resulting from the local electric field 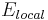 is given by:
is given by:
This introduces a complication however, as locally the field can differ significantly from the overall applied field. We have:
where P is the polarization per unit volume, and N is the number of molecules per unit volume contributing to the polarization. Thus, if the local electric field is parallel to the ambient electric field, we have:
Thus only if the local field equals the ambient field can we write:
Dispersion and causality
In general, a material cannot polarize instantaneously in response to an applied field, and so the more general formulation as a function of time is
That is, the polarization is a convolution of the electric field at previous times with time-dependent susceptibility given by 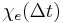 . The upper limit of this integral can be extended to infinity as well if one defines
. The upper limit of this integral can be extended to infinity as well if one defines 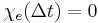 for
for 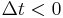 . An instantaneous response corresponds to Dirac delta function susceptibility
. An instantaneous response corresponds to Dirac delta function susceptibility 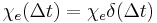 .
.
It is more convenient in a linear system to take the Fourier transform and write this relationship as a function of frequency. Due to the convolution theorem, the integral becomes a simple product,
This frequency dependence of the susceptibility leads to frequency dependence of the permittivity. The shape of the susceptibility with respect to frequency characterizes the dispersion properties of the material.
Moreover, the fact that the polarization can only depend on the electric field at previous times (i.e. 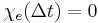 for
for 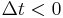 ), a consequence of causality, imposes Kramers–Kronig constraints on the susceptibility
), a consequence of causality, imposes Kramers–Kronig constraints on the susceptibility 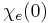 .
.
See also
- Application of tensor theory in physics
- Magnetic susceptibility
- Maxwell's equations
- Permittivity
- Clausius-Mossotti relation
- Linear response function
- Green–Kubo relations
References
- ^ "Electric susceptibility". Encyclopædia Britannica.
- ^ Cardarelli, François (2000, 2008). Materials Handbook: A Concise Desktop Reference (2nd ed.). London: Springer-Verlag. pp. 524 (Section 8.1.16). doi:10.1007/978-1-84628-669-8. ISBN 9781846286681. http://books.google.com/books?id=PvU-qbQJq7IC&pg=PA524&dq=Electric+susceptibility#v=onepage&q=Electric%20susceptibility&f=false.